

These periodic discharges often have larger amplitudes in comparison to the evoked potentials and can disturb the detection of cortical response. 6.6 Prognostication in Postanoxic Coma Bilateral absence of short-latency (N20) SSEP responses has been identified as the most powerful prognosticator of poor outcome in patients who remain. Sensory evoked potentials recorded from scalp electrodes represent electrical events generated along the sensory pathways. Thus, they provide data about the functions of specific areas of the nervous system in a noninvasive manner to determine the locus of alterations in sensory pathways.
Somatosensory evoked potential ( SEP or SSEP) is the electrical activity of the brain that results from the stimulation of touch. SEP tests measure that activity and are a useful, noninvasive means of assessing functioning. By combining SEP recordings at different levels of the somatosensory pathways, it is possible to assess the transmission of the from the periphery up to the cortex. SEP components include a series of positive and negative deflections that can be elicited by virtually any sensory stimuli. For example, SEPs can be obtained in response to a brief mechanical impact on the fingertip or to air puffs. However, SEPs are most commonly elicited by bipolar trancutaneous electrical stimulation applied on the skin over the trajectory of of the upper limb (e.g., the median nerve) or lower limb (e.g., the posterior tibial nerve), and then recorded from the scalp. In general, somatosensory stimuli evoke early cortical components (N25, P60, N80), generated in the contralateral (S1), related to the processing of the physical stimulus attributes.
About 100 ms after stimulus application, additional cortical regions are activated, such as the (S2), and the posterior and, marked by a parietal P100 and bilateral frontal N140. SEPs are routinely used in neurology today to confirm and localize sensory abnormalities, to identify silent lesions and to monitor changes during surgical procedures. Contents.History The modern history of SEPs began with George Dawson's 1947 recordings of somatosensory cortical responses in patients with, a neurological condition characterized by abrupt, involuntary, jerk-like contractions of a muscle or muscle group. Because of their relatively large amplitude and low frequency compatible with a low sampling rate of A/D conversion, the cortical SEPs were the first studied in normal subjects and patients. In the 1970s and early 1980s spinal and subcortical (far-field) potentials were identified. Although the origins and mechanisms of far-field SEPs are still debated in the literature, correlations among abnormal waveforms, lesion site, and clinical observations are fairly well established.
However, the most recent advances were brought about by multichannel recordings of evoked potentials coupled with source modeling and source localization in 3D images of brain volume provided by (MRI).Theory/source Modeling sources from the field distribution results in models of brain activation that may substantially differ from the observations of clinical correlations between the abnormal waveform and the lesion site. The approach based on clinical correlations supports the idea of a single generator for each SEP component, which is suitable for responses reflecting the sequential activation fibers and synaptic relays of the somatosensory pathways. Conversely, source modeling suggests that the evoked field distribution at a given moment may result from activities of multiple distributed sources that overlap in time. This model fits better with the parallel activation and the feedback controls that characterize the processing of somatosensory inputs at the cortical level.
Component characteristics. Way of nerve signal transduction and electrode positions for tibial (left) and median (right) nerve SEP recordingsWhen recording SEPs, one usually seeks to study peripheral, spinal, brainstem, and early cortical SEPs during the same run. Electrodes placed on the scalp pick up both SEPs generated in the cortex and thalamocortical fibers (which are picked up as near-field responses located in restricted areas) and far-field positivities reflecting the evoked activity generated in peripheral, spinal and somatosensory fibers.The literature is filled with discussions about the most appropriate site for the reference electrode to record each of the components. Considering the field distribution, the optimal recording condition is in theory that in which the reference is not influenced by the activity under study. Most of the far-field potentials are widely distributed over the scalp.
Consequently, they reach their maximal amplitude when the reference electrode is non-cephalic. A non-cephalic reference common to all channels is adequate for all near-field recordings. One relevant issue is that electrical physiological (electrocardiogram, electromyogram, etc.) noise level increases with the distance between the active and reference electrodes in non-cephalic reference montages. The routine four-channel montages proposed in the International Federation of Clinical Neurophysiology (IFCN) guidelines explore the afferent peripheral volley, the segmental spinal responses at the neck and lumbar spine levels, as well as the subcortical far-field and early cortical SEPs, using scalp electrodes placed in the parietal and frontal regions for upper limb SEPs and at the vertex for lower limb SEPs.Median nerve SEP begins with the delivery of an electrical stimulus to that nerve at the wrist.
A 100–300 microsecond square wave electrical pulse is delivered at intensitiesstrong enough to cause a 1–2 cm thumb twitch. Upon delivery of such a stimulus, nerve action volleys travel up sensory fibers and motor fibers to the shoulder, producing a peak as they enter. This peak is formally known as N9. In the course of conduction, the sensory fibers then transverse the cervical roots and enter the cervical cord. The pathway then joins the posterior columns, sending off collateral branches to synapse in the midcervical cord. This midcervical cord activity gives rise to a peak known as N13.
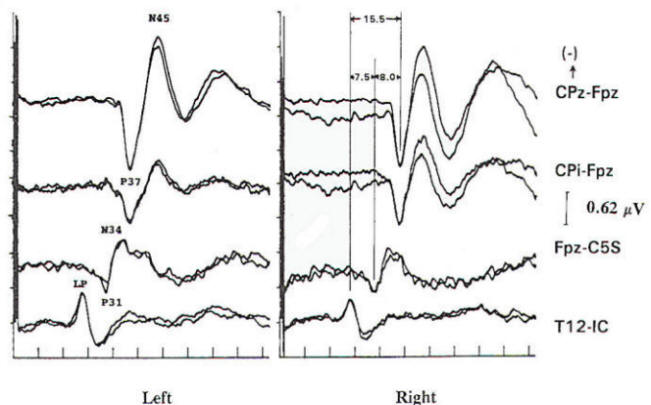
The N13 is best measured over the fifth cervical spine. Further conduction in the posterior columns passes through the synapse at the cervicomedullary junction and enters the lemniscal decussation. A scalp P14 peak is generated at this level. As conduction continues up the to upper midbrain and into the thalamus, a scalp negative peak is detected, the N18.
After synapsing in the and traversing the, the N20 is recorded over the contralateral to the wrist stimulated, corresponding to arrival of the nerve impulses at the primary somatosensory region.Posterior tibial nerve stimulation at the ankle gives rise to a similar series of subsequent peaks. An N8 potential can be detected over the posterior tibial nerve at the knee. An N22 potential can be detected over the upper, corresponding to the collateral activity as the sensory fibers synapse in the lumbar spinal cord. More rostrally, a cervical potential can occasionally be detected over the mid- or upper. Finally, a P37 scalp potential is seen over the midline scalp lateral to the midsagittal plane, but ipsilateral to the leg stimulated. Functional sensitivity Non-pathological factors The effects of age on SEP latencies mainly reflect conduction slowing in the peripheral nerves evidenced by the increase of the N9 component after median nerve stimulation. Shorter central conduction times (CCT, the transit time of the ascending volley in the central segments of the somatosensory pathways) have also been reported in females as compared to males, and conduction velocities are also known to be affected by changes in limb temperature.
It has always been assumed that cortical SEPs peaking before 50 ms following stimulation of the upper limb are not significantly affected by cognitive processes. However, Desmedt et al. (1983) identified a P40 potential in response to target stimuli in an, suggesting that attention-related processes could affect early cortical SEPs. Finally, some changes in the amplitude, waveform, and latency of the parietal N20 have been reported during natural sleep in normal subjects. Pathological factors Median and posterior tibial SEPs are used in a variety of clinical settings.
They can detect, localize and quantify focal interruptions along the somatosensory pathways, which may be due to any number of focal neurological problems, including trauma, compression, tumor or other focal lesions. SEPs are also sensitive to cortical attenuation due to diffuse (CNS) disorders. This is seen in a variety of neurodegenerative disorders and metabolic problems such as deficiency. When a patient suffers from sensory impairment, and when the clinical localization of the sensory impairment is unclear, SEPs can be helpful in distinguishing whether the sensory impairment is due to CNS problems as opposed to peripheral nervous system problems. Median nerve SEP is also helpful in predicting neurological sequelae following: if the cortical N20 and subsequent components are completely absent 24 hours or more after the cardiac arrest, essentially all of the patients go on to die or have vegetative neurological sequelae. Clinical applications. SEP recording of median nerveIn the recent decade, the clinical usefulness of SEPs entered the operating room, allowing the intraoperative monitoring of the CNS and, thus, safeguarding CNS structures during high risk surgeries.
Continuous SEP monitoring can warn a surgeon and prompt intervention before impairment becomes permanent. Testing with median nerve SEPs is used to identify the sensory and motor cortex during craniotomies and in monitoring surgery at the midcervical or upper cervical levels. Posterior SEP monitoring is widely used for monitoring the spinal cord during scoliosis procedures and other surgical interventions in which the spinal cord is at risk for damage. Recording of far field intracranially generated peaks can facilitate monitoring even when the primary cortical peaks are impaired due to anesthetic agents. Over time, SEP testing and monitoring in surgery have become standard techniques widely used to reduce risk of postoperative neurologic problems for the patient.
Continuous SEP monitoring can warn a surgeon about potential spinal cord damage, which can prompt intervention before impairment becomes permanent. Overall, SEPs can meet a variety of specific clinical objectives, including:. to establish objective evidence of abnormality when signs or symptoms are equivocal;. to look for clinically silent lesions;. to define an anatomical level of impairment along a pathway;. to provide evidence about the general category of the pathology;. to monitor objective changes in the patient's status over time.Experimental paradigms Besides the clinical setting, SEPs have shown to be useful in distinct experimental paradigms.
Schubert et al. (2006) used SEPs to investigate the differential processing of consciously perceived versus unperceived somatosensory stimuli. The authors used an 'extinction' paradigm to examine the connection between activation of S1 and somatosensory awareness, and observed that early SEPs (P60, N80), generated in the contralateral S1, were independent of stimulus perception. In contrast, amplitude enhancements were observed for the P100 and N140 for consciously perceived stimuli. The authors concluded that early activation of S1 is not sufficient to warrant conscious stimulus perception. Conscious stimulus processing differs significantly from unconscious processing starting around 100 ms after stimulus presentation when the signal is processed in parietal and frontal cortices, brain regions crucial for stimulus access into conscious perception.
In another study, Iwadate et al. (2005) looked at the relationship between physical exercise and somatosensory processing using SEPs. The study compared SEPs in athletes (soccer players) and non-athletes, using two oddball tasks following separate somatosensory stimulation at the median nerve and at the tibial nerve. In the athlete group the N140 amplitudes were larger during upper- and lowerlimb tasks when compared to non-athletes. The authors concluded that plastic changes in somatosensory processing might be induced by performing physical exercises that require attention and skilled movements.See also. ^ Mauguiere, F (1999). 'Somatosensory evoked potentials'.
Niedermeyer & F. Lopes da Silva (ed.). Electroencephalography: basic principles, clinical applications and related fields.
Williams and Wilkins. ^ Nuwer, Marc R (February 1998).
'Fundamentals of evoked potentials and common clinical applications today'. Electroencephalography and Clinical Neurophysiology.
106 (2): 142–148. Desmedt, John E; Nguyen Tran Huy; Bourguet, Marc (October 1983). 'The cognitive P40, N60 and P100 components of somatosensory evoked potentials and the earliest electrical signs of sensory processing in man'. Electroencephalography and Clinical Neurophysiology.
56 (4): 272–282. Nuwer, Marc R. 'Spinal Cord Monitoring With Somatosensory Techniques'.
Journal of Clinical Neurophysiology. 15 (3): 183–193. Schubert, Ruth; Blankenburg, Felix; Lemm, Steven; Villringer, Arno; Curio, Gabriel (January 2006). 'Now you feel it-now you don't: ERP correlates of somatosensory awareness'. 43 (1): 31–40. Iwadate, Masako; Mori, Akio; Ashizuka, Tomoko; Takayose, Masaki; Ozawa, Toru (7 December 2004).
'Long-term physical exercise and somatosensory event-related potentials'. Experimental Brain Research. 160 (4): 528–532.
SSEPs are usually evoked by bipolar transcutaneous electrical stimulation applied on the skin over the selected nerve and registered with disc electrodes along the tract. For example, in recordings of the median nerve, registration electrodes can be placed at the elbow, Erb’s point (over the plexus, above the clavicle) and cervical, parietal and frontal cortex (Fig.; see also Chap. Cortical responses can only be interpreted reliably when the peripheral responses are present. In the nomenclature of SSEP waveforms, N or P followed by an integer is used to indicate the polarity (positive, respectively, negative) and the nominal poststimulus latency (in ms) of the recorded wave in a healthy reference population (e.g. The earliest cortical potential is the N20, which is generated in the primary somatosensory cortex, where thalamocortical cells undergo synaptic connections with the superficial and deep pyramidal cell layers (Allison et al. In comparison to cortical responses of greater latency, the N20 is the most robust, as this is the latest waveform to disappear following increasing levels of encephalopathy or pharmacological sedation; of note, however, the N20 is relatively independent to the level of sedation used in clinical settings (Cruccu et al. Since the cortical waveforms appearing later (such as P45, N60 and P/N100) are less reliable and more susceptible to changes by sedation, the N20 is widely used in almost all clinical prognostic questions.
One of the main problems of the SSEP interpretation is the interobserver agreement, which has been extensively described in several studies (Zandbergen et al.; Pfeifer et al. Zandbergen et al. Investigated 56 consecutive patients with anoxic–ischaemic coma (Zandbergen et al. ); these registrations were interpreted independently by five experienced clinical neurophysiologists. The interobserver agreement for SSEPs in anoxic–ischaemic coma was only moderate (kappa 0.52, 95% CI 0.20–0.65): the main source of disagreement was related to the underlying electrical noise, implying difficulties in obtaining a reasonable signal-to-noise ratio. For recordings with a noise level of 0.25 μV or more, the mean kappa was as low as 0.34 (fair agreement), while for recordings with noise levels below 0.25 μV, the mean kappa improved to 0.74, which is a substantial agreement. Similar results have been reported by Pfeifer et al., again in subjects admitted after cardiac arrest (Pfeifer et al.
One way of integrating the SSEP information with other neurophysiological variables, in order to limit the aforementioned problems, may be a continuous SSEP registration combined with continuous EEG. This can be used to monitor deterioration in patients with severe brain injury (Amantini et al.
); however, such approaches are still rare in clinical practice. Of note, almost all literature regarding the use of SSEP for prognostication uses the absence or presence of short-latency cortical responses (N20). Whether the amplitude of cortical responses can be used is uncertain. During continuous SSEP and EEG registration, an N20 amplitude. Efforts should be made to increase the signal-to-noise ratio as much as possible. As an orienting threshold, Zandbergen et al. Recommend that the peak-to-peak amplitude of noise of the cortical and cervical leads should be lower than 0.25 μV after averaging, especially in the frequency of the SSEPs themselves (20–500 Hz) (Zandbergen et al.
Administration of muscle relaxants (together with modest doses of sedation, such as 5 mg of midazolam) often improves the quality of the SSEP registrations in patients with abundant muscle activity. Furthermore, disturbing electrical ICU equipment should be turned off as much as possible. Also, delivering more stimuli (up to 1,000 or more) and increasing the stimulus intensity could contribute to optimize the signal-to-noise ratio. In addition, it has been also suggested that the stimulus rate may influence the results of an SSEP recording (Robinson and Micklesen ) (see Chap. Since the interpreting clinician is often not present during the actual SSEP registration itself, the role of the technician is crucial in obtaining reliable results. Cortical responses are generally not influenced by moderate pharmacological sedation or metabolic disturbances, factors that often hamper the clinical neurological examination in the ICU. However, massive intoxications, very severe biochemical or metabolic disturbances and anatomical (e.g.
Cortical Somatosensory Evoked Potentials Comandante
A high cervical) lesions should be actively ruled out. The cortical N20 responses may remain still visible even at sedation levels sufficient to induce an isoelectric EEG (Cruccu et al.; Rothstein ); nevertheless, care should be taken when high-dosed barbiturates are administered: high-dosed sodium thiopental induces an increase in latencies and decrease in amplitudes for median nerve SSEP and brainstem auditory evoked responses (Drummond et al. It is uncertain whether the amplitudes decrease to a level that the cortical response can no longer be identified. Patients with absent cortical responses during thiopental (or pentobarbital) coma prescribed to treat increased intracranial pressures who made a good recovery in the end have been reported in the literature (Robe et al. This suggests that very high-dosed barbiturates can depress SSEP cortical responses. Propofol produces minimal suppression of the SSEP amplitude, which at most may be quantified to a loss of less than 10% (Langeron et al.
Somatosensory Evoked Potential During Surgery
Also, midazolam and opioids have only moderate effects on SSEP amplitudes and latencies (Langeron et al.; Asouhidou et al.; Laureau et al.; Taniguchi et al. Remifentanil can lower the cortical components by 20–80% when given at a high dose (0.8 mcg/kg/min) as used during neuromonitoring in the operating room (Asouhidou et al. On the other hand, as stated above, in some cases it may be even advisable to administer low-dose sedation to improve the quality of the SSEP recordings.
This is especially the case in patients with generalized periodic discharges on the EEG (see Chaps., and ), which in some situations can be suppressed after the administration of propofol. These periodic discharges often have larger amplitudes in comparison to the evoked potentials and can disturb the detection of cortical response. Bilateral absence of short-latency (N20) SSEP responses has been identified as the most powerful prognosticator of poor outcome in patients who remain unconscious after a circulatory arrest (Rossetti et al.; Bouwes et al. In patients not treated with hypothermia, bilateral absence of cortical N20 responses 24 h or more after the event represents a reliable predictor for a poor neurological outcome (which is understood as no recovery of awareness) (Zandbergen et al. A recent systematic review of all SSEP registrations reported in patients admitted to the ICU after resuscitation from a cardiac arrest and treated with hypothermia showed a false-positive rate (FPR) as low as 0.007, with a 95% confidence interval of 0.001–0.047 (Kamps et al.
These registrations were performed after return of normothermia. Even registration during therapeutic hypothermia might already have a solid good prognostic value, but the confidence interval is wider (Tiainen et al.; Bouwes et al.
